Presently, there are no drugs that can do this - existing ones just help with symptoms.
Biogen says it will soon seek administrative endorsement in the US for the "momentous" medicate, called aducanumab.
It plans to document the administrative work in mid-2020 and has its sights on Europe as well.
Endorsement processes could take a year or two. In the event that successful, the organization aims to at first offer the medication to patients previously tried out clinical studies of the medication.
The declaration is somewhat surprising because the organization had discontinued work on the medication in March 2019, in the wake of disappointing preliminary results.
In any case, the organization says another analysis of a bigger dataset of the same studies shows that higher doses of aducanumab can furnish a significant advantage to patients with early Alzheimer's, slowing their clinical decrease so they preserve a greater amount of their memory and consistently living skills - things that the disease usually robs.
Huge expectation
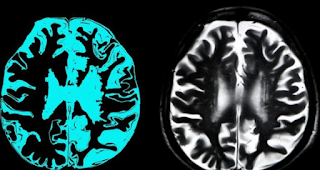
Aducanumab targets a protein called amyloid that forms anomalous deposits the brains of individuals with Alzheimer's. Scientists think these plaques are poisonous to synapses and that clearing them using drugs would be massive development in dementia treatment, in spite of the fact that not a fix.
There haven't been any new dementia drugs in over 10 years.
Biogen's CEO Michel Vounatsos said: "We are cheerful about the prospect of offering patients the first treatment to diminish the clinical decrease of Alzheimer's disease."
Hilary Evans from Alzheimer's Research UK said: "Individuals influenced by Alzheimer's possess sat tight along energy for a life getting updated new treatment and this energizing declaration offers new expectation that one could be in sight.
"Looking again at aducanumab is a positive step for each one of those who participated in the clinical trials and the overall dementia research network. As more information emerges, we trust it will spark worldwide discussions about the subsequent stages for conveying genuinely necessary treatments into individuals' hands."
Prof Bart De Strooper, Director of the UK Dementia Research Institute at University College London, said: "It is fantastic to know about these new positive results rising up out of the aducanumab trials. We as of now have no compelling treatments to slow or stop the progression of Alzheimer's disease and I trust this signifies a defining moment."
What is Alzheimer's?
Dementia is not a single disease, yet it is the name for a gathering of symptoms that incorporate problems with memory and thinking.
There are lots of various types of dementia and Alzheimer's is said to be the most widely recognized and most researched.
There are at present 850,000 individuals with dementia in the UK.
It's been a long and tortuous voyage to discover new drugs for the disease and ongoing attempts have finished in disappointment.
Experts trust a treatment is in sight, yet they are cautious and should closely scrutinize these aducanumab preliminary findings.
No comments:
Post a Comment